Network for innovation and health
The Danish-German border region has a wealth of skills and resources to develop new innovative ideas, technologies and products in the health sector. Nowadays, traditional innovation processes do no longer automatically lead to success.
The project Access & Acceleration strikes a new path and utilises the key success factor of innovations: a strong integration of technology users, companies and universities throughout all development stages.
News
Access & Acceleration results are online
After three years, Access & Acceleration is coming to an end. All results from the individual teams of the project are now published on the publications page:
https://www.accessinnovation.eu/publications.html
Results of the Access & Acceleration final conference
The final conference took place on 10 February 2022 in a virtual meeting. Project results and their integration into the Access & Acceleration platform were presented. Participants received ideas for the development of new business ideas and cooperations in the fields of education, research and innovation.
Access & Acceleration brochure available
The project partners of Access & Acceleration have developed a brochure on the most important results of the project. The brochure is available in three languages as a PDF download:
- German: Access_Projektbroschüre.pdf
- Danish: Projektbrochure.pdf
- English: Access_Project_brochure.pdf
Invitation to Network Meeting
Due to the currently increasing level of Covid19, we need to refrain from our planned network meeting on 24 November, unfortunately.
We thank you very much for your interest and would like to offer you to meet up individually with our partners instead. In order to schedule an appointment, please contact Katharina Rubahn (kru@tek.sdu.dk; T +45 65 50 12 24).
On 24. November 2021 the project partners cordially invite you to a network meeting at the University of Southern Denmark in Sønderborg. Now that the pandemic situation allows personal meetings again, Access & Acceleration would like to take the opportunity to enter into a further personal dialogue on health innovation.
The meeting shall be used as an advisory platform on the Access & Acceleration project activities and provide you with:
- an update on the project’s outcomes, especially on the platform on health innovation,
- insights into ongoing industry-academy and clinic-academia collaboration projects,
- the possibility of a guided tour to the SDU’s premises as well as
- a dialogue on future joint initiatives.
The meeting will take place from 10.0-12.30 at Alsion, Sønderborg.
Please click on the following link for programme details and registration: https://www.conferencemanager.dk/network-meeting
Project partner Roana de Oliveira Hansen wins 2021 TEK Prize
Project partner Roana Oliveira Hansen received University of Southern Denmark’s TEK Innovation prize 2021. Roana works at NanoSYD, the nanoscience centre located at the Mads Clausen Institute of the University of Southern Denmark (SDU). The case study New sensor for clean water – functionalized surfaces is part of the Access & Acceleration project in which Roana examined the development of innovative new sensors ensuring quality and safety of food and water. In collaboration with the Danish company AmiNIC, she developed sensors to minimise meat and fish waste throughout the value chain.
‘Roana has a talent for creating binding synergies between companies and academia and has demonstrated how your research can be relevant for high-tech products,’ emphasizes Søren Elmer Kristensen, Head of TEK Innovation at the SDU in his congratulatory speech. Watch the video with the full congratulatory speech from Søren below.
Learn more about the collaboration on the waste water project at our Access & Acceleration platform: https://www.access-platform.eu/en/2021/05/06/new-sensor-for-clean-water-functionalized-surfaces-2/
TEK Prize 2021 - TEK Innovation Prize
Ali Ebrahimi successfully defensed his doctoral thesis
Although Alcohol Use Disorder (AUD) causes a large amount of morbidity, mortality and injuries every year, many patients suffering from AUD never undergo specialist treatment during their addiction due to a variety of reasons. How can this problem be solved? What is the best solution to build a predictive model for classifying patients into Normal, Harmful, and Hazardous drinkers? And how can patients with AUD be detected at an earlier stage? Project partner Ali Ebrahimi from the SDU Health Informatics examined these and other questions in his doctoral thesis with the title Predictive Models to Identify Patients with Alcohol Use Disorder, which has now been successfully defensed. Ali Ebrahimi studied at the SDU and researched on the topics Health Care, Machine Learning, Data Mining, Data Analysis and Natural Language Processing. Congratulations to Ali on his successful defense!

Web seminar: How to foster medical innovation?

Access & Acceleration aims to promote innovation in the health sector and to open up the innovation process to all stakeholders - from universities to industry and practising physicians to the patients themselves. For this purpose, we have developed an online platform for companies and hospitals for exchange and match making. In addition, we bring together relevant stakeholders in workshop and seminars.
Open innovation involves different stakeholders who can positively contribute to the innovation process by bringing in their perspectives and expertise. This allows new ideas and technologies to flow into the process and increase its potential.
On June 14, we organised the web seminar "Potentials and challenges of open innovation in healthcare”. More than 30 international guests participated. Prof. Dr Carsten Schultz from Kiel University gave a keynote on current research results in innovation management in the healthcare sector, highlighting the complex innovation ecosystem, the benefits and challenges of university-industry collaboration and fundamental hurdles such as the “not-developed-here”-phenomena. Afterwards Prof. Dr Uffe Wiil from University of Southern Denmark presented the results from the Patient@home project and addressed challenges in data-driven health technology.
In the following round table discussion we were able to welcome in addition Dr Thomas M. Helms (Chairman of the Board of the German Foundation for the Chronically Ill), Uwe Herrmann (Consultant for Innovation and Technology Management), and Dr Niels Reimers (Global Director R&D of Stryker).
"All in all, we can take home two conclusions from this event: Considering patients as stakeholders of innovation is essential, because they know their diseases and what they need for an optimal treatment. Furthermore, trust between the innovation partners is the key to success," says project partner Till Leißner from the Southern Danish University in Sønderborg.
A roadmap for successful market entry
How can innovative medical products be brought to market? What are the market entry barriers in Germany and Denmark? The project partners from the Center for Innovativ Medicinsk Teknologi, the Mærsk Mc-Kinney Møller Institute and the University of Lübeck have published a roadmap to help users successfully enter the market.
Web seminar - Potentials and challenges of open innovation in healthcare
Which factors are essential for the innovation process? How can we find innovative solutions to problems in the health sector together with stakeholders from the health sector? The project partners of Access & Acceleration will discuss these and other questions with you in their web seminar.
The web seminar will take place on 14 June from 16:30-18:00, event language is English. Registration is free of charge and possible via the event website until 10 June.
Read more about the event and registrate at the event website: https://event.sdu.dk/openinnovation/
Accelerating innovation - the Access & Acceleration platform is online
The Access & Acceleration partners have been working on a German-Danish innovation platform for the health sector. In different teams, the partners have taken a closer look at the different stages of the innovation cycle: from developing new ideas in the health sector, to testing the development of products and services on the basis of two pilots and the market entry.
The platform enables users to network with each other and enter into cross-border collaborations to jointly implement innovative ideas and technologies in the health sector. Now the platform is published and freely accessible.
"The Access & Acceleration platform was built and developed to use the existing innovation potential from the two different health systems more effectively. Our platform accompanies the user along the innovation cycle and provides him with methods for the specific management of innovation processes," says project partner Katharina Rubahn from the University of Southern Denmark.
Learn more about the Access & Acceleration platform by clicking the following link:
https://www.access-platform.eu
In the following video Katharina Rubahn explains how the platform can be used:
Explanatory video about the Access platform
Our video shows what it could look like if our Access & Acceleration platform is used to launch medical innovations in the German-Danish border region.
Click here for the Danish version on YouTube.
Prediction of patients with alcohol use disorder from historical electronic health records
During a virtual opening of the Centre for Clinical Artificial Intelligence (CAI-X), project partner Ali Ebrahimi from the SDU Health Informatics presented his study A Predictive Machine Learning Model to Determine Alcohol Use Disorder. The study covers an iterative exploratory methodology to develop an accurate predictive model to identify patients with alcohol use disorder (AUD) from historical electronic health records (EHR). The study demonstrates how machine learning algorithms are employed to diagnose a disorder based on EHRs of 2,534 patients admitted at the Odense University Hospital in the region of Southern Denmark. The main goal of this research is to develop a predictive model that can be used in the hospital decision support system platform empowering hospital staff to take early action against alcohol-related problems in patients. To achieve this goal, several predictive models will be developed and tested to make the use of already collected patient data better accessible for medical staff. This study has been developed within the Access & Acceleration project. Learn more about the study at doi: 10.1109/ISCC50000.2020.9219685

Ideas campaign for better aftercare of fracture patients
How can we improve the aftercare of patients with fractures? The project partners of Access & Acceleration addressed this question to physiotherapist who provide ambulatory or stationary care to get to know more about their challenges in the aftercare of fracture patients.
The answer revealed three central topics that play an essential role:
- involvement of physiotherapists in the holistic aftercare process
- activation of patients through training, participation and codecision
- planning and quality assurance of the physiotherapeutic treatment
As part of the ideas campaign “Ideas from physiotherapists for physiotherapists”, the project partners now want to work with physiotherapists to develop concrete solutions tailored to the identified topics. The project partners will be supported by the innovation consulting and software company Nosco, which provides an online platform where ideas can be collected, evaluated and further developed. Nosco will also to advise on the ideas campaign.
The Nosco online platform will promote the digital exchange and collaboration. With optimised workflows and processes the platform enables to identify the best ideas and thus, to increase the likelihood that these will also be implemented.
The ideas campaign is organised by the project partner Kiel University in cooperation with the German Institute for Therapy Research and Access & Acceleration network partner Buchner & Partner GmbH.
The German business magazine up also covers the issue of the idea campagne in a separate article.
Click here to read the full article in the online edition of the up magazine (in German).

Online survey on market barriers
The project partners from Access & Acceleration have developed an online survey to find out how to facilitate the entry to the healthcare market for German and Danish companies.
The survey is available in German and Danish and addresses companies of the healthcare industry to find out more about possible market entry barriers. Click on the following link to participate in the survey:
German survey https://www.survey-xact.dk/LinkCollector?key=RW5FXADPL615&lang=3.0
Danish survey https://www.survey-xact.dk/LinkCollector?key=RW5FXADPL615&lang=2.0
The survey has been created by the project partners Maersk Mc-Kinney Moller Institute, Centre for Innovative Medical Technology and University of Lübeck, Clinic for Orthopaedics and Trauma Surgery.
Online survey on the impacts of the COVID-19 pandemic
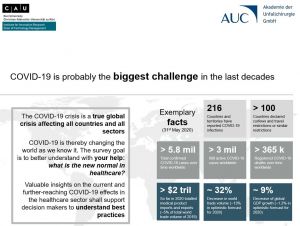
How will our healthcare system change due to the COVID-19 pandemic? The project partners have elaborated two online surveys to tackle the question on the influence of the COVID-19 pandemic on various trends within the health care system. One survey addresses medical professionals, the other employees of the health industry (medical technology, biotech, pharma).
If you belong to one of the groups, please support us and participate in the survey via the following links:
- Survey for medical professionals: https://ww3.unipark.de/uc/COVID-19-survey2020/
- Survey for employees of the health industry: https://ww3.unipark.de/uc/COVID-19-survey/
The surveys were developed by the Access & Acceleration project partners Kiel University and Stryker Trauma GmbH and supported by the European trade association MedTech Europe Group, Deutsche Gesellschaft für Unfallchirurgie and Akademie der Unfallchirurgie GmbH.
Virtual seminar on the impacts of the COVID-19 pandemic on the healthcare system
On 18 August 2020, our network partner Life Science Nord will host a virtual seminar entitled “The ‘New Normal’ within the healthcare system”. During this seminar, Prof. Dr Carsten Schultz from Kiel University and project partner Dr Nils Reimers, Stryker Trauma GmbH, will discuss the development of the COVID-19 pandemic and how it will influence our future healthcare system. The seminar is free of charge and takes about one hour (11:00 - 12:00 h). You can join it via the following link: https://www.edudip.com/de/webinar/lsn-spotlight-das-new-normal-im-gesundheitswesen/387548
Comparing German and Danish healthcare systems
The health systems of Germany and Denmark differ fundamentally from each other. A lack of knowledge of these differences can make it difficult or even impossible for medical companies to enter the market. The project partners from the Mads Clausen Institute of the South Danish University (SDU) have compiled an overview that lists the main differences between the two health systems. You can download the document as a PDF here: https://accessinnovation.eu/files/Content/News/Health%20Care%20Systems_en.pdf
Interview with the Access & Acceleration project advisory board

The project advisory board supports Access & Acceleration during the whole project lifetime. With its expert knowledge and constructive impulses from the perspective of potential users and stakeholders, the advisory board members contributes to the strategic development of the project. We have talked to Anna-Marie Bloch Münster from the Hospital of South West Jutland and Jonas Drefeld from Health Innovation Center of Southern Denmark – both members of the project advisory board- about the perspectives and potentials of the Access & Acceleration.
What would you say are the most interesting aspects of the Access & Acceleration project?
Anna-Marie Bloch Münster: For me, this includes: The development of new technologies that could offer a broader spectrum of treatment options (e.g. the treatment of smaller tumors which are difficult to access); greater safety during treatment through increased precision and the development of less invasive techniques that minimise complications for patients.
Jonas Drefeld: I am glad that the project intends to increase the focus on user needs by redesigning existing development processes in way that users (such as patients, employees from various areas of the health sector, companies) become an integral part of development activities. I especially look forward to see new insights and methods on how public and private actors can co-create user-centered medical technology without compromising current legislation. I am furthermore confident that the strong project partnership develops a sustainable innovation platform that can run without subsidies after the project period.
What do you hope will be the impact or added value from the Access & Acceleration project? Who will benefit from the project?
Anna-Marie Bloch Münster: In my opinion, it is the patients who benefit the most. The added value for them should be an increased safety during the therapy of diseases and minimised side effects. Ultimately, it should also be a goal to develop new techniques that enable the treatment of previously incurable diseases. In addition, the project will of course add value for physicians as they will be able to get new treatment techniques on hand and will be able to offer patients safer and less invasive therapies.
Jonas Drefeld: I hope that the project creates new methods for how implementation can be an integral part of the development processes of new health care technologies. In my opinion, the project would be a success if it manages to develop methods and strategies for effective adoption and scaling of technologies globally. This knowledge would enable companies and healthcare providers to implement the suitable technology that benefits healthcare workers and citizens.
Are there also other German-Danish projects you are currently involved in or were involved in? If yes, which ones?
Anna-Marie Bloch Münster: No, not yet.
Jonas Drefeld: The Health Innovation Center was a part of the German-Danish health project Demantec during its first phase. The project aim was to develop, test and implement e-Health solutions that addresses user needs for persons suffering from dementia, their relatives and healthcare workers. The Health Innovation Center tested, redesigned and implemented two technologies that improved the care and quality of life for nursing home residents suffering from dementia. Insights from the project are gathered in a go-to implementation guide for assisted living technologie
Working on overcoming market barriers
Due to the COVID-19 pandemic, the third Access & Acceleration project meeting, on April 30th, 2020 took place online. The partners from the Centre for Innovative Medical Technology (CIMT), the Maersk Mc-Kinney Moller Institute SDU (MMMI) and the University of Lübeck (UZL) jointly presented their results on identifying market access barriers in Germany and Denmark. It is notoriously difficult to access the Danish and German markets for healthcare products with new technological solutions. It is especially hard for SMEs that do not possess the necessary knowledge and market insight.
One of the main objectives of the partners from CIMT, MMMI and UZL is to provide access to an ecosystem which members know the healthcare sectors and have clear market access strategies. Within the Access & Acceleration project, they focus on three main activities:
- Improvement the innovation dialogue for identifying market access barriers in Denmark and Germany;
- Development of strategies for access to markets in Denmark and Germany;
- Validation and dissemination of market access strategies.
The participatory approach of the partners involves in-depth interviews and workshops with relevant stakeholders on the German and on the Danish side, to reveal the kind of methods and knowledge they need to be able to enter the respective market. Preliminary results indicate that there are multiple barriers where the most significant derives from the difference in healthcare structure. While in Denmark it is public funded, the healthcare system in Germany is financed by social security contributions from all health insurers.
The lack of knowledge about the differences in legislation and the new medical device regulation are further market barriers. Additionally, there is also the language barrier since both Germany and Denmark provide information on their healthcare legislation only in their native language.
The next steps for CIMT and UZL will be to develop strategies on how to support the different stakeholders in understanding the health care structure, legislation and how to overcome language barriers to create a common understanding of the respective healthcare system.
Scoping Review on the prediction of alcohol use disorder
How to support medical staff in evaluating patient data? The project partners from the Centre for Innovative Medical Technology (CIMT) want to tackle this issue by developing a software which can store patient data and, based on that, predict the course of a disease. As a case study, CIMT uses patient data related to alcohol use disorder (AUD). In this context, CIMT published a review of literature which focused on the prediction of AUD. A link to an abstract of this review can be found in the publication’s section of the project website at: https://www.accessinnovation.eu/publications.html

How to support companies from the healthcare sector in entering the market?

Project partner Florian Ege tells us about the main activities and Welfare Tech’s biggest challenges in the Access & Acceleration project.
Which are your main activities within the Access & Acceleration project?
Welfare Tech is a Danish national cluster and hub for innovation and business development in healthcare, homecare and social services. Within the project, we see our main role in promoting the project’s activities to private companies from the region. We are therefore mainly involved in the activities around project communication, but also in the ideation process and market barrier analysis.
Which of the project aims imposes the biggest challenge from your point of view?
The German and Danish healthcare systems are characterised by fundamental differences in setup and design. Understanding these differences and communicating them to involved stakeholders such as private companies will pose a considerable hurdle. Thereafter, it will be crucial to support said stakeholders in understanding the other healthcare system and transform gained insights into actionable points allowing for product development and market access.
How will Access & Acceleration change the present situation in the health sector of your region?
As a member-driven cluster organisation Welfare Tech currently experiences an especially strong interest of Danish and Nordic companies for the German market. This appears to be driven on the one side by push factors such as the uncertainties around the British Brexit negotiations and pull factors such as recent digitalization efforts made by German healthcare legislators. Germany offers great opportunities for Danish companies to scale and expand and the Access & Acceleration project seeks to support these activities.
First project workshop completed successfully
On November 19th, 2019 a workshop with the topic on market access for medical device in Germany and Denmark took place. The participants from clinics, research and industry discussed their own experiences and got inputs from the healthcare project Digital Health & Care 4.0, led by the Danish cluster organisation WelfareTech. Digital Health & Care 4.0 focused on questions about the organisation of the German healthcare sector and useful strategies for companies to enter the German market. Generally, the results of the workshop indicate that diverging national regulations and laws as well as completely different structured healthcare systems in Germany and Denmark are major obstacles when it comes to entering the German market. Solutions to tackle this issue and to support relevant stakeholders of the German and Danish healthcare sectors to overcome these obstacles will be a crucial aim of the Access & Acceleration project.
The project partner Centre for Innovative Medical Technology (CIMT) organised this workshop in Odense, Denmark. Within the project they are responsible for identifying barriers of entry on the market regarding new health technologies and products, but also for developing strategies to overcome these barriers.
Click here to stay tuned for the next workshop!

Kick-off in Sønderborg
At their first official meeting on 28 May in Sønderborg, Denmark, the German and Danish project partners of Access & Acceleration came together to set the course for the future cooperation. The aim of the six project partners is to establish a cross-border platform that will enable key players in the healthcare sector to connect and collaborate with each other in order to initiate innovative processes.
Interreg approves project application: Access & Acceleration up and running
The Interreg committee has approved the Access & Acceleration project application. The Interreg programme Deutschland-Danmark will support the project with around 1.7 million Euros over the next three years.
More information on: https://www.interreg5a.eu/blog/neues-29-mio-euro-schweres-eu-projekt-im-gesundheitswesen/
Newsletter archive
Newsletter No. 5 – December 2021
Newsletter No. 3 – January 2021
Newsletter No. 1 – January 2020
Visit us on our social media pages: